Real-World Data (RWD) and Real-World Evidence (RWE) have shown an increasing usage throughout the last decade (1). The technological advancements are responsible for the enhancement in computer software and their storage capacity. However, much still remains to be developed. The awareness of this approach is still extremely unknown to regulatory agencies stakeholders. The investment and growth discussed can be visualized extremely well in the post-marketing trials sector (1), as it can be seen below. This graphic depicts the number of post-marketing clinical trials in which have been applied RWD. The rising use of this application throughout 2008 to 2016 shows a difference of 3700 clinical trials which is also indirectly a proof of the benefits that can be brought in clinical trials setting. However, some improvements are still bound to be made.
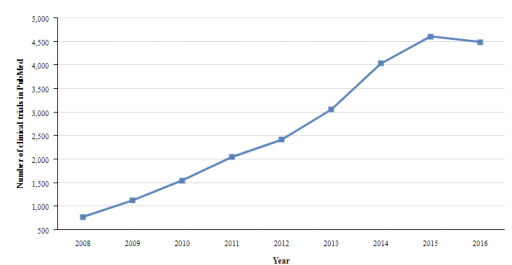
Source: https://druginnovation.eiu.com/real-world-data-trials/
In order to fully make use of this data and evidence, it is necessary to outline one major aspect: security and data protection as a means to safely secure people’s data and for it to be only used for clinical trials and other research purposes. Therefore, it is fundamental to invest in the creation of regulated databases that store and secure all distinct types of data, allowing most diseases and other research information to be easily accessed without violating an individual's privacy or prior consent policies.
Indeed, several advances are notoriously being developed worldwide. For instance, the “FAIR Guiding Principles for scientific data management and stewardship” movement (2) focus on sharing scientific data while following 4 principles: Findability, Accessibility, Interoperability and Reuse where scientists share their discoveries in more accessible methods. This has the objective to rely on Artificial Intelligence (AI) approaches due to the excessive volume of data nowadays, which consequently mitigate the possibility of biased data in a research that was discussed in the challenges of the use of this data. Another example of rare diseases database development is the National Organization for Rare Disorders (NORD) database (3) where it is shown a broad range of information of various rare diseases. These types of projects are augmenting RWD quality as well as optimizing the application of RWE as a factor to be applied in all future regulatory decision-making processes regarding clinical trials.
In addition, It is important to give a perspective of the various developments being done in different parts of the world. On the one hand, it is worthwhile to mention a few European projects being one an ongoing work towards the growth in genomic sequence storage, which is a type of RWD (4). Another european initiative is the development of databases specifically regarding rare disease in which the stored information takes into consideration different aspects that are fundamental to the growth of knowledge in the rare disease field such as mechanism study, mutated gene location discoveries and other general information regarding specific rare diseases like CDG (4). To reach this goal, one task force, EU1MG, is trying to identify potential pilot projects within the rare diseases field.
On the other hand, initiatives like these can also be found in the US. The Food Drug Administration (FDA) (5) has also ongoing projects to amplify the influence of RWD in the clinical world. Projects such as building data analytics platforms that are trying to “provide an integrated database and analytics hub designed to promote the secure sharing of existing patient-level data and encourage the standardization of new data collection.” which is one major challenge that RWD encounters.
In conclusion, RWD and RWE have been experiencing a steady growth and a extremely promising one. These approaches seem to be an innovative procedure in the clinical spectrum which assists in optimization of clinical trials, such as speeding up their duration. Moreover, the creation of more organized databases is helping to augment the efficiency of RWD assessment and the overall knowledge of different rare diseases with the accumulation of the RWD simultaneously. However, it is important to point out that some challenges are yet to overcome; for instance, the lack of awareness from regulatory decision-making committee members as it was underlined before. The future looks auspicious for RWE and RWD application as a key factor in the regulatory decision-making process, this is supported by the results of Deloitte survey where the majority of the respondents believes in the increasing importance of RWD in R&D in the next two years (7). This is of extreme importance for the CDG community due to the fact that clinical trials are emerging in the field of CDG. Thus, it urges to raise awareness about RWD and RWE within the CDG community, using different strategies:
- identification of resources aimed at educate the CDG community
- creation of this section at the web-based platform WorldCDG.org devoted to RWD and RWE;
- Administration of an electronic questionnaire to identify major perceptions, needs and potential solutions from CDG stakeholders.
Bibliography
- https://druginnovation.eiu.com/real-world-data-trials/ Acessed March 2021
- https://www.go-fair.org/fair-principles/ Acessed March 2021
- https://rarediseases.org/ Acessed March 2021
- Horgan D, Bernini C, Thomas PPM, Morre SA.(2019). Cooperating on Data: The Missing Element in Bringing Real Innovation to Europe's Healthcare Systems. Public Health Genomics. 22(3-4):77-101. doi: 10.1159/000503296. Epub 2019 Oct 21. PMID: 31634895; PMCID: PMC6943808.
- https://www.fda.gov/news-events/fda-voices/rare-disease-day-2021-fda-shows-sustained-support-rare-disease-product-development-during-public Acessed March 2021
- Polak TB, van Rosmalen J, Uyl – de Groot CA. Expanded Access as a source of real-world data: An overview of FDA and EMA approvals. Br J Clin Pharmacol. 2020;86:1819–1826. https://doi.org/10.1111/bcp.14284
- https://www2.deloitte.com/us/en/insights/industry/health-care/real-world-evidence-study.html Accessed March 2021
In this section:
Real World-Data & Evidence
Didn't find what you are looking for?
Your question may help others
Authors
Alexandre Gil and Pedro Granjo from Sci and Volunteer Program Nova School of Science and Technology 2021.
Disclaimer
The Site cannot and does not contain medical or health advice. The information is provided
for general informational and educational purposes only and is not a substitute for
professional advice.
Accordingly, before taking any actions based upon such information, we encourage you to
consult with the appropriate professionals. We do not provide any kind of medical or health
advice. The use or reliance of any information contained on this site is solely at your own
risk.
Follow Us
Like the World CDG Organization Facebook Page. Share the page on your own timeline, and tell your friends to share it.
Follow us on Twitter and LinkedIn.
Subscribe to our Youtube channel and invite your friends to subscribe too.

