The following section was created using several sources of information considered as reliable and transferable for CDG. Choosing whether or not to participate in a clinical trial is a decision that millions of individuals make each year. Before making that decision, it is important to learn about clinical trials and what it means to be a study participant. The frequently asked questions below help you learn about and what to consider before taking part in a trial.
To access additional resources for information about clinical trials for people living with CDG and their families move for other website sections.
The U.S. Department of Health and Human Services video provides basic information about informed consent and what information you’ll get to help you decide whether to volunteer for a research study.
Frequently Asked Questions
- What is a Clinical Research?
- How do you get started on the road to clinical research participation?
- What are the potential benefits of participating in a clinical trial?
- What are the possible risks of participating in a clinical trial?
- Why are clinical trials important?
- Who can participate in a clinical trial?
- What is informed consent?
- What are the phases of clinical trials?
- How is the safety of the participant protected?
- Who manages clinical trials?
- Does the participant pay to be in a clinical trial?
- Will I be paid and how will I receive my payment?
- Can I drop out of the clinical trial if I need or want to?
- What is a placebo?
- What happens if I receive the placebo?
- What is a control or control group?
- What is randomization?
- What are single-blind and double-blind studies?
- What is a protocol?
- What happens when a clinical trial is over?
1. What is a Clinical Research?
Clinical research refers to studies in which people participate as patients or healthy volunteers. Different terms are used to describe clinical research, including:
- clinical studies
- clinical trials
- studies
- research
- trials
- protocols
Further reading here. Watch the video below produced by the National Institute of Child Health and Human Development (NICHD) to learn how you can participate and contribute to medical advances.
Carefully conducted clinical trials are the fastest and safest way to find treatments that work in people and ways to improve health. Observational studies do not test new drugs or treatments. In observational studies, doctors and researchers analyze health data to find links between a diagnosis and certain health conditions, disease progression, symptoms or quality of life.Interventional studies test new ways to prevent, detect, or treat diseases. Treatments may be new drugs, combinations of drugs, surgical procedures, medical devices, or lifestyle interventions like diet and exercise. Learn more here.
Clinical research is much different from the medical treatment you receive in a Healthcare Provider's office. Learn about the differences at the FDA section Clinical Research Versus Medical Treatment.
To continue to read:
- The Center for Information and Study on Clinical Research Participation (CISCRP) has made available a video entitled General Clinical Research Overview, which walks you throughout (1) the overview of clinical trials and the process, (2) the different study phases, meanings and timelines and (3) safeguards in place when participating in a clinical trial. Watch it below
- Another resource led by CISCRP is the video dedicated to Basics of Clinical Trial Participation.
- ThePancreasPatient created a video that explains what clinical trials are, how they are conducted, and why they are important for patients with diseases like pancreatic cancer. Overall relevant concepts are explained. Watch here.
2. How do you get started on the road to clinical research participation?
CISCRP produced the brochure Should I Participate and an informative interactive infographic paves the way with a Clinical Research Participation Basics Roadmap. Download it here.
3. What are the potential benefits of participating in a clinical trial?
- You may gain access to new drugs and other treatments, sometimes years before they are widely available.
- You will be monitored closely for any side effects.
- You will have the chance to take an active role in your own healthcare.
- You will be making a valuable contribution to cancer research.
To continue to read:
- Clinical Trials and Me prepared a simple video where the possible benefits and risks of participating in a clinical trial are explained. Watch here.
4. What are the possible risks of participating in a clinical trial?
- A clinical trial can sometimes require more time and medical attention than normal care. This can include doctor visits, phone calls, more treatments, a hospital stay, or a more complicated treatment regimen. (Ask your doctor for information about the trial you are considering.)
- The treatment might not work.
- The treatment might cause serious side effects.
- Even if a new approach helps some patients, it might not help you.
To continue to read visit the list of frequently asked questions about clinical trials for patients and their families made available by The Memorial Sloan Kettering Cancer Center here.
5. Why are clinical trials important?
Advances in medicine and science are the result of new ideas and approaches developed through research. New treatments must prove to be safe and effective in clinical trials with a certain number of patients before they can be made widely available.
Through clinical trials, researchers learn which approaches are more effective than others. This is the best way to test a new treatment. A number of standard treatments were first shown to be effective in clinical trials. These trials help us find new and better treatments.
For further reading:
- The UC San Diego Health website has a section about Research & Clinical Trials, where there is a list of frequently asked questions about clinical trials that also includes the answer for each question. Read here.
6. Who can participate in a clinical trial?
All clinical trials have rules about who can and cannot participate. These rules are called “eligibility criteria". The criteria are based on factors such as age, gender, the type and stage of a disease, previous treatment history, and other medical conditions. Eligibility criteria is not used to reject people personally. Instead, the eligibility criteria helps to make sure that the participants in the trial are safe and that the trial gets the most accurate information about the treatments being researched. Some clinical trials seek participants with certain illnesses or conditions, while others need healthy participants. Continue reading the section created by the National kidney foundation here.
7. What is informed consent?
Informed consent is the process of learning about a clinical trial before agreeing to participate. To help you decide if you want to participate, the doctors and nurses involved in the trial explain the details of the study. They will provide you with an informed consent document that includes details about the study, such as its purpose, duration, required procedures, and key contacts. Risks and potential benefits are explained in the informed consent document. You then decide if you want to sign the document and join the trial. Informed consent is not a contract—participation is always voluntary and you may end your participation at any time. The informed consent process ensures you have the facts you need before making a final decision.
To continue to read:
- EUPATI hosts a section dedicated to Informed Consent
- Melanoma Research Alliance (MRA) has put together answers to some of the most common questions that you may have. Read here.
The video below was created for an informed consent training workshop for research trainees at the University Health Network, and explains what informed consent is and challenges around it.
- The U.S. Department of Health and Human Services made available a video that provides basic information about informed consent and what o information you’ll get to help you decide whether to volunteer for a research study. Watch it below
8. What are the phases of clinical trials?
Most clinical research that involves the testing of a new drug progresses in an orderly series of steps, called “phases.” This allows researchers to ask and answer questions in a way that results in reliable information about the drug and protects the patients. Most clinical trials are classified into one of three phases:
Phase I trials: These first studies in people evaluate how a new drug should be given (by mouth, injected into the blood, or injected into the muscle), how often, and what dose is safe. A phase I trial usually enrolls only a small number of patients, sometimes as few as a dozen.
Phase II trials: A phase II trial continues to test the safety of the drug, and begins to evaluate how well the new drug works. Phase II studies usually focus on a particular type of cancer.
Phase III trials: These studies test a new drug, a new combination of drugs, or a new surgical procedure in comparison to the current standard of care. A participant will usually be assigned to the standard group or the new group at random (called randomization). Phase III trials often enroll large numbers of people and may be conducted at many doctors' offices, clinics, and cancer centers nationwide.
In addition, after a treatment has been approved and is being commercially marketed, the drug's maker may study it further in a phase IV trial. The purpose of phase IV trials is to evaluate the side effects, risks, and benefits of a drug over a longer period of time and in a larger number of people than in phase III clinical trials. Thousands of people are involved in a phase IV trial.
Several resources are publicly available such as
- Mayo Clinic created the video below intended to explain The Clinical Trial Journey.
- The European Federation of Pharmaceutical Industries and Associations (EPFIA) made available an explanatory video devoted to The Journey of Clinical Trials.
- Antidote made available an infographic named What Happens in Each Clinical Trial Phase. Download here.
- Read and share the infographic produced by NIH to learn why researchers do different kinds of clinical studies. Download here.
- Watch the following video created by National Cancer Institute to learn about the three phases of clinical trials.
- Complex stories helped make the pathway for ushering new drugs from research, to development, to trial, to approval, clear in the following colorful, playful explanation infographic . View here.
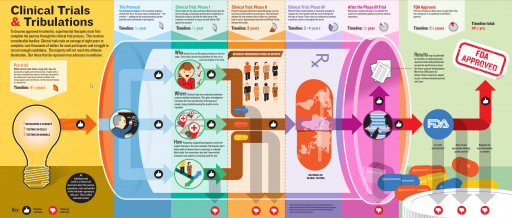
- The infographic created by “clinicaltrialsgps” named Understanding the Phases of Clinical Trials shows the entire process of a clinical trial broken up into four phases. Download here.
- The Nordic Trial Alliance made an illustration showing the progression of a clinical trial available here.
- Checkout the explanatory Clinical trial process done by Cystic Fibrosis Foundation here
To continue to read:
- The FDA prepared a section dedicated to What Are the Different Types of Clinical Research? and to Clinical Research
- The UC San Diego Health website has a section about Research & Clinical Trials, where there is available a list of frequently asked questions about clinical trials that also includes the answer for each question. Read here.
9. How is the safety of the participant protected?
The ethical and legal codes that govern medical practice also apply to clinical trials. In addition, most clinical research is federally regulated with built in safeguards to protect the participants. The trial follows a carefully controlled protocol, a study plan which details what researchers will do in the study. As a clinical trial progresses, researchers report the results of the trial at scientific meetings, to medical journals, and to various government agencies. Individual participants’ names will remain secret and will not be mentioned in these reports.
Each trial has scientific oversight, and people living with a certain condition also have rights that help protect them. The National Cancer Institute has made available a video explaining the rules in place to help ensure the safety and ethics of clinical trials in the USA. Watch below:
Overall Patient Safety includes careful review and approval of the clinical trial protocol by:
- Institutional Review Board (IRB): Watch the video produced by the U.S. Department of Health and Human Services on How IRBs Protect Human Research Participants.
- The informed consent process
- Scientific experts
Ongoing monitoring of the trial by:
- The IRB
- Data and Safety Monitoring Boards (DSMBs) for phase 3 trials
- The organization sponsoring the trial
- The research team
To continue to reading:
- The National Institute on Aging (NIA) section dedicates a section to Clinical Trials: Benefits, Risks, and Safety
- The Clinical center study hosts a section dedicated to How Do Clinical Trials Protect Participants
- The European Patients Forum (EPF) created a fact sheet available here
10. Who manages clinical trials?
Each clinical trial is managed by a research team that can include doctors, nurses, research assistants, data analysts, and other specialists. The research team works closely with other health professionals, including other doctors and nurses, laboratory technicians, pharmacists, dietitians, and social workers, to provide medical and supportive care to people who take part in a clinical trial.
The research team closely monitors the health of people taking part in the clinical trial and gives them specific instructions when necessary. To ensure the reliability of the trial’s results, it is important for the participants to follow the research team’s instructions. The instructions may include keeping logs or answering questionnaires. The research team may also seek to contact the participants regularly after the trial ends to get updates on their health. For further reading consult Bon Secours website that hosts a Clinical Trials Portal where you can find a list with frequently asked questions about clinical trials. Read here.
11. Does the participant pay to be in a clinical trial?
The costs for the treatments and tests in the trial are usually covered by the research sponsor. This can include the treatment being studied and other treatments, such as blood tests, physical examinations, and imaging scans like X-rays. However, there can be costs that are not covered by the sponsor. These may include time away from work, travel to and from the research site, child care coverage during study appointments, etc. In some cases, the research sponsors will provide trial participants with compensation to help pay for these costs. People who are considering participating in a trial can ask the research staff about any costs before agreeing to participate in a trial.
12. Will I be paid and how will I receive my payment?
Yes. You’ll be paid for your time and participation during the clinical study. The payment you receive varies from study to study, depending on its length and type. Payment details will be discussed with your study team at your first visit.
For further reading:
- EUPATI made available a presentation describing the compensation in clinical trials, which can be adapted for own use here. You can access also to An overview of the different models that help set the amount of compensation participants receive for taking part in a clinical trial.
- TrialFacts hosts a section named A Quick Guide to Participant Compensation in Clinical Trials
13. Can I drop out of the clinical trial if I need or want to?
Yes, absolutely. Research coordinators discuss this process at length during the consent process. If you drop out, the research coordinator will likely ask that you complete a termination visit and any safety follow-up visits to ensure you do not experience any side effects. To continue to read, the Crohn’s & Colitis Foundation has made an educational resource (an infographic) dedicated to Clinical trials FAQs available here.
14. What is a placebo?
Placebos are harmless, inactive substances made to look like the real medicine used in the clinical trial. Placebos allow the investigators to learn whether the medicine being given works better or no better than ordinary treatment. In many studies, there are successive time periods, with either the placebo or the real medicine. In order not to introduce bias, the patient, and sometimes the staff, are not told when or what the changes are. If a placebo is part of a study, you will always be informed in the consent form given to you before you agree to take part in the study.
For further reading:
- Ted-Ed made available a video The power of the placebo effect created by Emma Bryce
- The CISCRP created a brochure intended to help you understand what ‘placebos’ are, and why they are used in clinical trials. Download here.
15. What happens if I receive the placebo?
This is a question that we hear a lot. Typically, participants are not told what treatment they are being given until a clinical trial is over. So, participants will not know if they are receiving the study drug or placebo during the trial. Research coordinators and trial staff themselves often do not know which participants are receiving the study drug or placebo. This helps reduce possible biases and ensures the fairness of the trial.
All participants, whether on a placebo or the treatment, are monitored very closely during the clinical trial. If there is any change in your medical condition while participating in the study, the research staff will inform you immediately and discuss the situation. For example, if you are a participant that receives a placebo and your condition does not improve or your symptoms worsen, the research staff can recommend that you stop the trial or that you talk with your doctor about receiving another medical treatment. Whichever group you are in, you can expect to receive excellent medical care during the trial. For further reading here.
16. What is a control or control group?
The control group is defined as the group in an experiment or trial that does not receive treatment. The control group is then compared to the group receiving the study treatment to see if the treatment is working.
For further information
17. What is randomization?
Randomization is a process used in some clinical trials to prevent bias. Bias occurs when a trial's results are affected by human choices or other factors not related to the treatments being tested. Randomization helps ensure that unknown factors do not affect trial results.
Randomization is used in some phase II and all phase III trials. These trials are called “randomized clinical trials.” If you participate in such a trial, you will be assigned by chance to either an investigational group or a control group. Your assignment will be determined with a computer program or table of random numbers.
- If you are assigned to the control group, you will get the most widely accepted treatment (standard treatment or "standard of care").
- If you are assigned to the investigational group, you will get the new treatment being tested.
Comparing the control and investigational groups often clearly shows which treatment is more effective or has fewer side effects. If you are thinking about joining a randomized clinical trial, you need to understand that you have an equal chance to be assigned to either one of the groups. The doctor does not, and cannot, choose the group for you.
To continue to read:
- The UC San Diego Health website has a section about Research & Clinical Trials, where there is available a list of frequently asked questions about clinical trials that also includes the answer for each question. Read here.
For further information:
- The U.S. Department of Health and Human Services created a video that explains the concept of randomization in research studies and what potential participants need to know when volunteering for a study with a randomized design. Watch below.
18. What are single-blind and double-blind studies?
In single- or double-blind studies, the participants don’t know which medicine is being used, and they can describe what happens without bias. Blind studies are designed to prevent anyone (doctors, nurses, or patients) from influencing the results. This allows scientifically accurate conclusions. In single-blind (“single-masked”) studies, only the patient is not told what is being given. In a double-blind study, only the pharmacist knows; the doctors, nurses, patients, and other health care staff are not informed. If medically necessary, however, it is always possible to find out what the patient is taking. To continue to read visit, Clinical Partners, LLC has made an educational resource dedicated to Clinical trials FAQs available here.
19. What is a protocol?
A protocol is a study plan that was carefully designed to safeguard the health of the research volunteers as well as to answer specific research questions. A protocol defines the types of people who may participate in the trial, the schedule of tests, procedures, medications and dosages, and the length of the study. To continue to read, Shepherd Center is a private, not-for-profit hospital that offers a list of frequently asked questions about clinical trials and all the answers on their website. You can find it here.
20. What happens when a clinical trial is over?
After a clinical trial is completed, the researchers look carefully at the data collected during the trial to understand the meaning of the findings and to plan further research. After a phase I or phase II trial, the researchers decide whether or not to move on to the next phase or stop testing the intervention because it was not safe or effective. When a phase III trial is completed, the researchers analyze the data to determine whether the results have medical importance and, if so, whether the tested intervention could become the new standard of care.
The results of clinical trials are often published in peer-reviewed scientific journals. Peer review is a process by which cancer research experts not associated with a trial review the study report before it is published to make sure that the data are sound, the data analysis was performed correctly, and the conclusions are appropriate. If the results are particularly important, they may be reported by the media and discussed at a scientific meeting and by patient advocacy groups before they are published in a journal. Once a new intervention has proven safe and effective in a clinical trial, it may become a new standard of care.
The FDA Amendments Act of 2007 requires that researchers report the “basic” results of clinical trials that tested FDA-regulated and FDA-approved chemical or biologic agents or medical devices in ClinicalTrials.gov, a publicly accessible database maintained by the U.S. National Library of Medicine (NLM). Basic trial results must be submitted whether or not the results are published in a peer-reviewed scientific journal and include the following items:
- Demographic and baseline information about the participants
- The progress of the participants through each stage of the trial (e.g., the number of participants who left the trial and at which stage)
- Results for the primary and secondary outcomes (also called endpoints) measured in the trial (e.g., tumor response, disease-free survival, overall survival, quality of life, etc.)
- A point of contact for the trial (to obtain additional information about the trial and its results)
For further reading check the full reference:
- Bon Secours created a Clinical Trials Portal where you can find a list with frequently asked questions about clinical trials. Read here.
Additional resources for information and frequently asked questions about clinical trials for people living with a condition and their families can be found at:
- The Crohn’s & Colitis Foundation made available an educational infographic compiling FAQs. Download here
- The National Human Genome Research Institute has developed a Clinical Research FAQ
- The EPF created a factsheet Putting the Patient at the centre of Clinical trials
- The Sphere offers a FAQs about Clinical Trials
- The Memorial Sloan Kettering Cancer Center has made available a list of frequently asked questions about clinical trials for patients and their families that also includes all the answers. Read here.
- CSL Limited is a leading global biotech company that explains the answers for the most frequently asked questions about clinical trials in a page of their website. Read here.
- Covance by labcorp carries out clinical research studies on behalf of the world's leading pharmaceutical and biotechnology organizations. Covance offers a list where a variety of frequently asked questions are answered one by one. Read here.
- Clinical Partners,LLC is a research site conducting clinical trials in Johnston, Rhode Island on the Atwood Medical Center Campus. An educational resource dedicated to Clinical trials FAQs ia available here.
- The Office for Human Research Protections (OHRP) hosts an educational section on Human Research Volunteer Informational Videos here
Author
Marta Silva Sci and Volunteer Program FCT NOVA 2021.
Disclaimer
The Site cannot and does not contain medical or health advice. The information is provided for general informational and educational purposes only and is not a substitute for professional advice.
Accordingly, before taking any actions based upon such information, we encourage you to consult with the appropriate professionals. We do not provide any kind of medical or health advice. The use or reliance of any information contained on this site is solely at your own risk.
Follow Us
Like the World CDG Organization Facebook Page. Share the page on your own timeline, and tell your friends to share it.
Follow us on Twitter and LinkedIn.
Subscribe to our Youtube channel and invite your friends to subscribe too.

